When discussing the safety of breast implants, it’s important to know that few medical devices—if any—have been studied more than silicone gel breast implants. I’ve served as a clinical investigator for cohesive silicone gel implants and have published clinical research detailing more than 1,000 silicone implant case studies. I can confidently say that women getting breast implants at my Los Angeles practice have a very low risk of complications.
A low risk, of course, isn’t zero risk, and plastic surgeons should always discuss these risks with patients considering breast augmentation. That’s a responsibility I take seriously, and I think it will be helpful to discuss breast implant safety in this blog post. I’ll first provide a brief overview of the history of breast implants and then describe the two most-discussed safety issues: breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and breast implant illness (BII).
A Brief History of Modern Breast Implants
Since surgeons first used silicone gel breast implants for augmentation in the 1960s, manufacturers have continued to make improvements. Today, women undergoing breast augmentation surgery can choose from the 7th or 8th generation of silicone gel implants, which are more cohesive than ever before.
Saline implants became a popular alternative when the FDA restricted the use of silicone implants from 1992 to 2006. The FDA lifted those restrictions in 2006 when extensive studies demonstrated the safety of the implants. At the same time, the agency approved silicone implants for patients aged 22 and older. Since 2006, cohesive silicone gel implants have become increasingly popular, with about 85% of patients today choosing them instead of saline.
Today, breast augmentation surgery is safer than ever, thanks to advanced implant technology and new techniques, including those I use for rapid recovery breast augmentation. Women can rest assured that the FDA carefully monitors breast implant safety and ensures that those considering the procedure have all the knowledge they need to make well-informed choices.
The Safety of Today’s Breast Implants
According to the American Society of Plastic Surgeons, about 400,000 women in the United States get breast implants every year. Around 300,000 of those women choose breast augmentation for cosmetic reasons, and 100,000 choose implants to reconstruct their breasts after they have mastectomies to treat or prevent breast cancer. The vast majority of these women experience no health issues related to having implants.
The older the implants, however, the more likely a complication can occur. This complication may be leakage or a condition such as capsular contracture when the capsule of scar tissue surrounding the implant becomes harder and squeezes the device. Capsular contracture can distort the implant’s appearance and cause pain.
The safety concerns that have generated the most recent discussion, however, are BIA-ALCL and BII. Let’s take a closer look at each of these conditions.
What Is BIA-ALCL?
BIA-ALCL is cancer that affects the immune system and is, in most cases, found in the scar tissue and fluid near the breast implant. Even though it occurs in the breast, it is not breast cancer. The link between BIA-ALCL and breast implants was initially identified by the FDA in 2011, and ongoing studies have concluded that the risk of developing BIA-ALCL remains low.
Textured vs. Smooth Breast Implants
The surface of breast implants can be either smooth or textured. The majority of implants used in breast augmentation procedures are smooth, round implants. Anatomically shaped implants are textured to help keep them from rotating inside the implant pocket.
As the FDA continued to evaluate evidence of cases of BIA-ALCL in the U.S. and around the world, researchers found that the risk of BIA-ALCL is higher for textured implants versus smooth implants. The FDA specifically linked BIOCELL textured implants made by Allergan to the risk of developing BIA-ALCL. In July 2019, Allergan voluntarily agreed to remove these devices from the market. Because the risk was so low, the FDA did not recommend that women with these types of implants have them removed if they weren’t experiencing symptoms associated with BIA-ALCL.
BIA-ALCL Symptoms
Any patient experiencing the following symptoms should schedule an appointment with their doctor:
- Unexplained breast enlargement
- Pain
- Lump in the breast or armpit
- Rash
- Hardening of the breast
- A large accumulation of fluid
If you don’t have these symptoms and elect to keep your implants, you should still get an ultrasound or MRI exam every 2 years to ensure the integrity of the implants.
What Is BII?
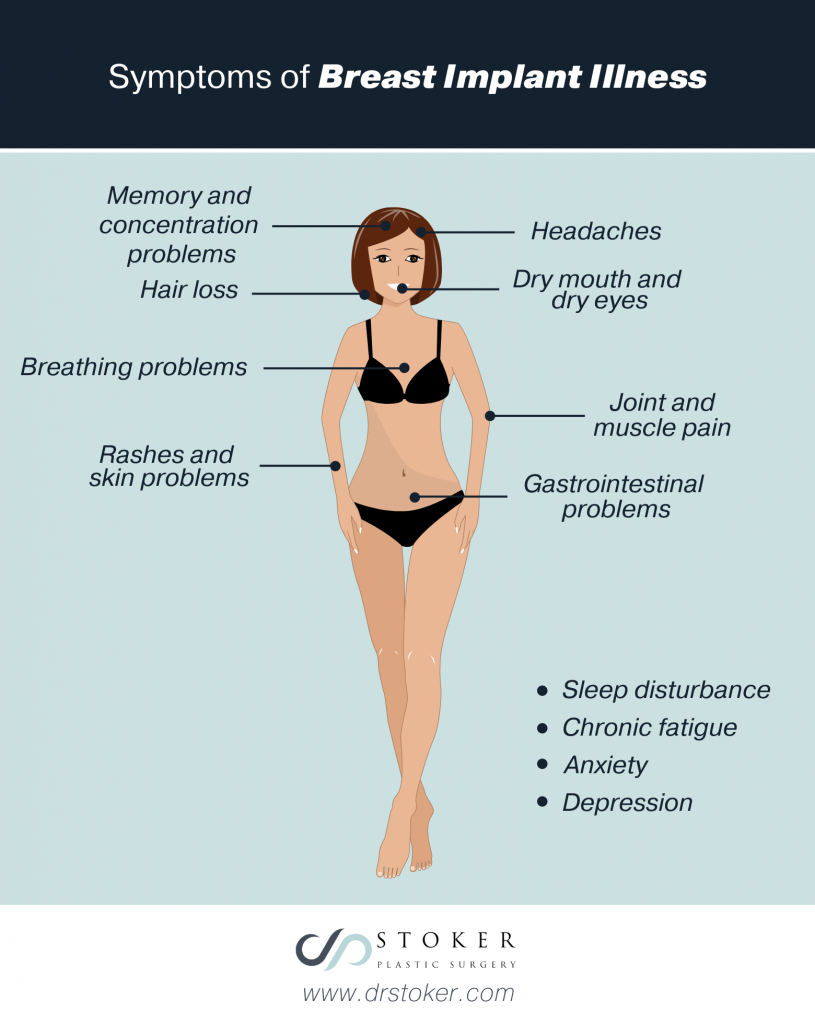
The emergence of BIA-ALCL has also highlighted an unrelated condition called breast implant illness, or BII. This condition includes a collection of physical and emotional symptoms that women believe are linked to their breast implants. Researchers evaluating the link between implants and women with these symptoms have not defined BII as a specific diagnosis. The symptoms may occur at any time after getting implants, including years later.
These symptoms include:
- Joint and muscle pain
- Chronic fatigue
- Memory and concentration problems
- Breathing problems
- Sleep disturbance
- Rashes and skin problems
- Dry mouth and dry eyes
- Anxiety
- Depression
- Headaches
- Hair loss
- Gastrointestinal problems
Even though it is uncertain whether breast implants cause these symptoms, several studies indicate that some women see most of their symptoms improve partially or completely after having their implants and capsules removed.
If You’re Concerned About BII
Your doctor may order blood tests to eliminate other possible causes because the symptoms of BII overlap with other conditions. If the tests rule out any other causes, the doctor may recommend implant removal.
If you choose to remove your implants, our practice fully supports that decision. Breast implant revision surgery is more complicated than the initial breast augmentation procedure, and it’s important to choose a board-certified plastic surgeon who has experience performing the revision surgery.
If you’d like to discuss your options at our Los Angeles plastic surgery practice, request a consultation using the online form or give us a call at (310) 300-1779 to schedule an appointment.


Leave a Reply